

Moreover, blue-green and violet are other lights that depicted their wavelengths within the ranges that are available in the literature. These wavelengths are closely within the range of 491nm and 575nm, a range for the green light (Grossie and Underwood 286). Green light has a wavelength of 540nm for argon, 570nm for krypton, 560nm for hydrogen and neon. Green, green-blue, and violet are some of the lights, which depicted their wavelengths to be consistent with literature values. The wavelengths of the yellow light are not within the range of 575nm to 585nm that is in literature except for neon (Grossie and Underwood 286). Neon, argon, and krypton produced a yellow light with wavelengths of 590nm, 490nm, and 480nm respectively. However, neon produced orange light with an experimental wavelength of 520nm, which is not within the normal range of 590 to 625nm. The wavelength is within the range of 625nm to 740nm for the red light. From the standard plot or calibration plot, red light showed experimental wavelength of 675nm in hydrogen and neon. Derived wavelengths from the calibration plot depicted the relationship between scale reading and the wavelengths of gases such as hydrogen, neon, argon, and krypton. The scale reading of the spectroscope displayed wavelengths that correlated with the standard wavelengths in terms of nanometer. However, the experiment did not present any new spectra lines. The procedure of the experiment deviated from printed procedures because the position of the graph-paper scale and observer is unique. In the experiment, when the observer viewed the diffracted light, red, yellow, green, blue-green, blue, and violet are sequential colors that are visible on the graph-paper scale.
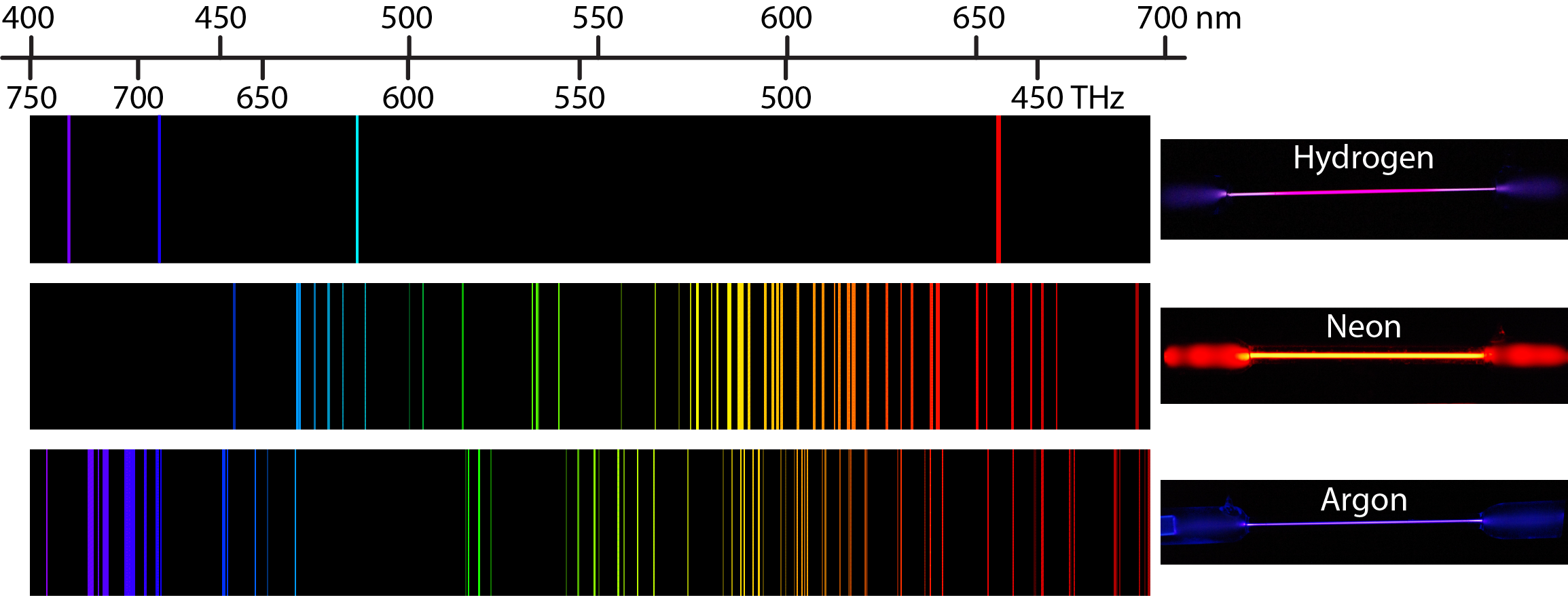
Since the beam of light diffracts as it leaves diffraction grating, an observer can view the diffracted light on a graph-paper scale, and note the readings. According to Grossie and Underwood, the slot is an entrance slit that allows 1mm of light beam to pass into the box and hits diffraction grating at an incidence angle of 30° (287). The spectroscope comprised of the cigar box, which has a slot, a source of light, a diffraction grating, and a graph-paper scale. The experiment used spectroscope to determine wavelengths of the visible colors. To determine unknown wavelengths using standard wavelengths, calibration plot is necessary. If the angle of diffraction (θ) and the distance between lines on a diffraction grating (d) are known, then one can calculate the wavelength using the equation (λ = dsinθ). The diffraction grating then diffracts the stream of light and separates it into atomic spectra according to their respective wavelengths. Spectroscope measures atomic spectra by generating a stream of light using a slit (entrance slit).Īfter generating stream of light using entrance slit, spectroscope then allows the stream of light to pass through diffraction grating (lens or prism). To measure atomic spectra, spectroscope is necessary. Since the change of quantum levels is intermittent, there is an emission of light in form of spectral lines. For example, when atoms or ions gain energy in the form of heat, electrons in them changes their quantum levels from ground state to an excited state. The formation of the atomic spectra occurs when atoms or ions intermittently absorb and emit energy due to the excitation of the electrons.


 0 kommentar(er)
0 kommentar(er)
